I am a chartered engineer and I’ve been doing this stuff for a long time. In the course of my career (if that’s the right term) I’ve come across a lot interesting projects and, generally, I’ve had an enjoyable time being an engineer, I like it. There are somethings I don’t particularly like, Project Managers for example.
There’s an old joke:
|
|
There’s a man flying in a hot air balloon and suddenly he realises he’s lost.
Reducing height, he spots another man on the ground and shouts “Excuse me Sir, can you tell me where I am?”
The man below says: “Yes. You're in a hot air balloon, hovering 30 feet above this corn field”.
“You must be an engineer” says the balloonist. "
“I am” replies the man. “How did you know?”
“Well” says the balloonist, “everything you’ve told me is technically correct, but It's of no practical use at all”.
The man below thinks for a while and then replies, “You must be a project manager”.
“I am” replies the balloonist, “How'd you know?”
“Well” says the man, “you don’t know where you are or where you’re going, but you expect me to help. You’re in exactly the same position you were before we met, but somehow now it’s my fault”.
|
I am that engineer.
Unfortunately, there is another class of manager that is even worse: Validation Managers. These are politely and respectfully referred to as “Felchers†” by engineers everywhere. They live in pharmaceutical companies (when they’re not in cloud cuckoo land) and are a right pain in the arse.
|
|
|
|
| † |
|
Felcher [noun, slang] A well-established term in the Engineer’s Lexicon of Insults.
Er… if you are of a squeamish nature, I shouldn’t look it up. |
They are the Jeremy Corbyn supporters of the pharmaceutical world: entirely convinced of their own moral superiority and completely dismissive of any other argument. They have a stifling certitude; an implacable self-righteousness and they are always, always willing to be offended (I imagine they would fit right in on social media).
The regrettable thing for engineers, is that these validation managers have some power (and by God are they pissed with it) and they can make life difficult if you don’t jump through their hoops.
The problem is, I want my software to be usable in pharmaceutical systems, after all a valve is a valve whether it’s in a pharmaceutical plant or a water treatment works, it opens and it closes. This means that the software has to be validated.
Validation, in terms of “it’s a validated system”, is the process of making sure a computerised system (such as a PLC and its software) does precisely what it was designed to do; specifically, it is the exercise of correctly and traceably documenting every requirement of the system and making sure that that requirement is formally and exhaustively tested.
If you haven’t come across the term before it probably means you haven’t been working in the pharmaceutical industry — but don’t worry, other industries are looking at it and starting to think it’s a good idea. Not only is it springtime for Hitler and Germany, it’s looking pretty good for the validation people too and some of them make Hitler look like a walk in the park.
If you are an engineer, validation will be coming soon, to a town near you! — It’s time to get dressed and saddle up.
Validation usually involves a lot of signatures in black ink and a lot of stern looks from the validation people (particularly if you don’t write the date the correct way), validation people don’t smile much in my experience, they are not party people — think Nurse Ratched
|
|
Two validation managers were asked to validate the company flag pole to make sure it matched the correct height requirements.
They go out to the flagpole with ladders, string and a tape measure, but they’re struggling to reach the top and can’t get the correct measurement.
Two engineers passing by see what they’re trying to do, the engineers walk over, lift the flagpole from its footings, lay it flat on the ground and measures it from end to end. The engineers put the flagpole back, give the measurement to the managers and walk away.
After they’ve gone, one validation manager turns to the other sniggering “Isn’t that just typical of engineers? We’re looking for the height and they gives us the length.”.
|
Validation itself is fairly straight forward; it is to some extent exactly what you would do yourself if you knew nothing about it. If you were writing some PLC software to do something, you would probably have a document that explained what the PLC was to do: open a valve when the level reaches this value; and you would probably write a document to test the function: did the valve open when the level reached that value?
Easy — this is what engineers have always done. We don’t just build something; we build it and then test it to make sure it works properly. We didn’t just build the Apollo rocket and fire it into space, we blew lots of them up first, figuring out how to fire it into space.
Engineers have always tested things, we’re conservative, we assume we’re going to cock it up the first few times and we test and we test until we get it right. Pharma people — not so much — think Thalidomide; Thalidomide was considered safe because they couldn’t find a dose high enough to kill a rat without squashing it and it sold in nearly the same quantities as aspirin before they realised something was wrong.
I understand why pharma people are nervous (once bitten and all that) and why they want to get it right, the nuclear industry does it for the same reasons (just watch Chernobyl if you want to see engineers really screw something up).
What I don’t like is the secrecy that surrounds validation, it’s a bit of a club where those in the club don’t really want to explain it to those outside; they say they do (because it’s supposed to be open and transparent), but they don’t. There’s lots of information but it’s all designed to be a bit intimidating and to make you feel, well, stupid. I found the same thing when I looked at Git and version control systems. It reminds me very much of the gramophone sketch by the Not the Nine O’clock News team. It’s the same attitude.
Lots of people make a living out of validating systems and they do so by overcomplicating it, everything is abbreviated (ICH Q9, GAMP, FEMECA) they use opaque terminology and jargon (component realisation, lifecycle support, white box, black box, pharmacovigilance), it’s how the medical and legal professions protect themselves from outsiders. Er… engineers aren’t blameless in this department either.
Validation itself is a good idea; it’s not a bad approach to take to every project — sometimes it’s required (pharma, nuclear, oil and gas), sometimes it’s completely over the top (PLCs playing the trombone†), but most projects benefit from the approach (even if they tone down the rigour and rules a bit; most projects don’t care how you write the date or make sure you draw a line through every blank area on a page).
|
|
|
|
| † |
|
When I worked for Siemens, we had a demonstration project to show the capabilities of a PLC. We decided to make one play “I’m forever blowing bubbles” on a trombone. It had an air compressor connected to the mouthpiece and a three-phase motor driving the slide and it was bloody loud (engineering at its finest). |
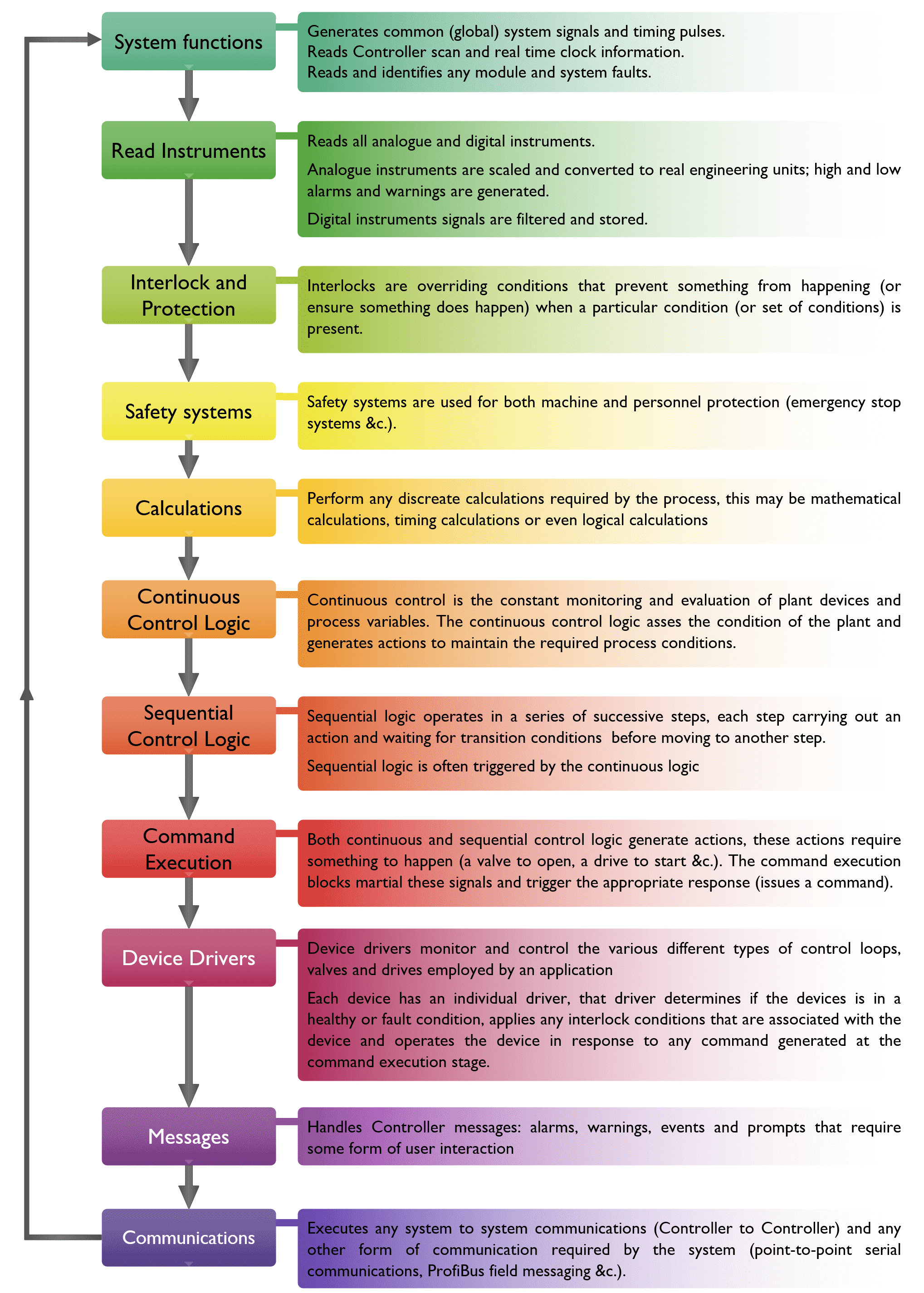
It also occurred to me at this point that the project itself, with all its documents, specifications, matrixes, test sheets and a practical approach to validation might be of use to some engineers too. It is a very good example of what documents are needed for a validated system, what should be in those documents and the correct form of wording to use.
So, I’ve made it all available I even explain how to structure all the folders on a hard drive and where to keep everything.
There is a complete copy of everything, including all the signed test sheets (with the date in the correct format to keep validation managers happy — happy is probably the wrong word).
Michael Gledhill
Chester—February 2025